Message from the Chair
Our department has a rich history of excellence in research, education and training.
Education
Explore the broad field of biochemistry with our BS and BA undergraduate programs. Or advance your career with our PhD and MS programs, as well as dual-degree programs that combine an MS in Biochemistry with JD, MBA, or MD degrees.

Who We Are
Our faculty members are leaders in a wide range of biomedical research areas.
News
“Can We Trust The Experts?”
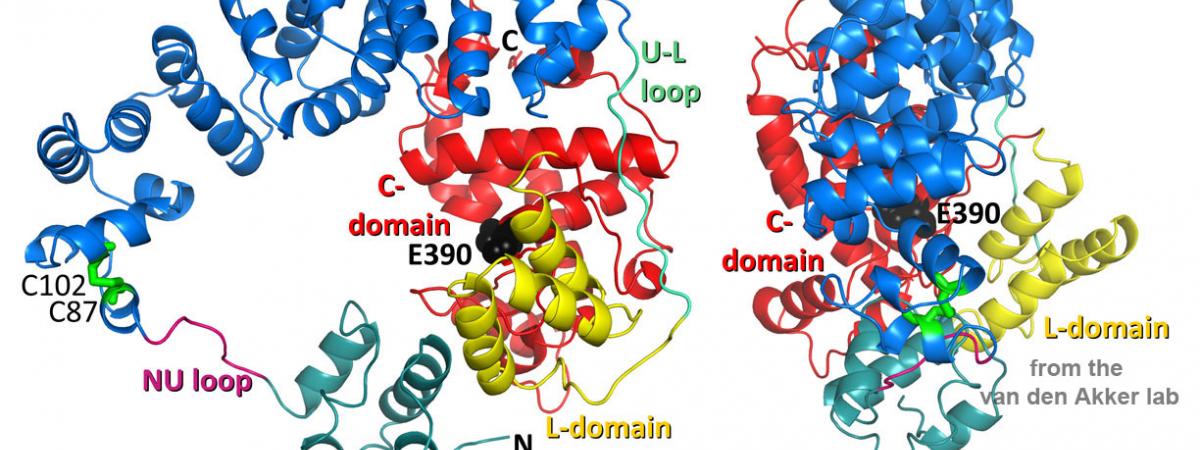
March 25, 2024Part of the Open Discourse Lecture & Discussion Series, Van den Akker will present “Can We Trust The Experts?” Monday, April 1, from 12:45 to 2 p.m. in Mather…Congratulations to Dr. Ryan Arvidson!
March 11, 2024We are very proud to share that our own Dr. Ryan Arvidson has been selected as a 2024 recipient of the Carl F. Wittke Award for Excellence in Undergraduate Teaching…Postdoc Neekkan Dey has work accepted into Molecular Cancer Research Journal
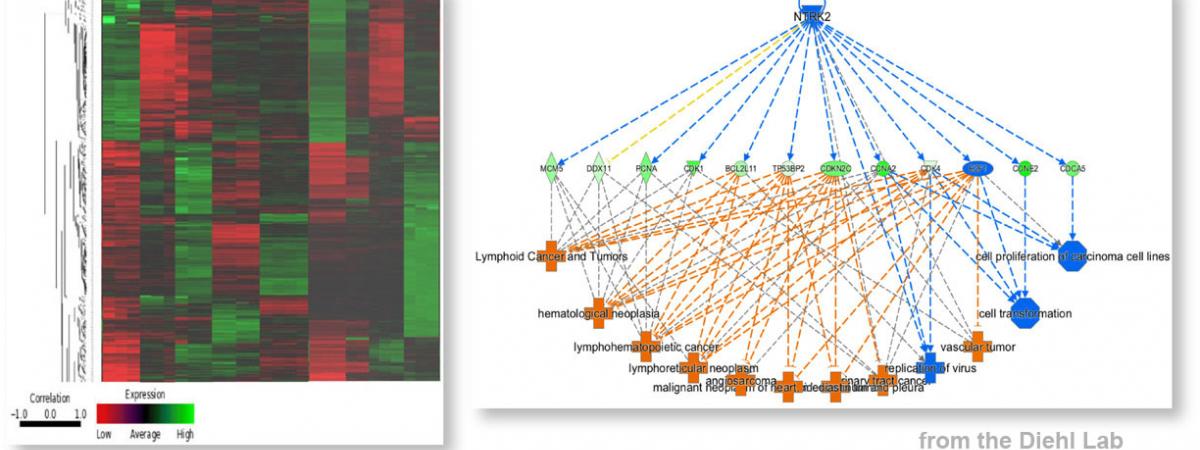
February 14, 2024Neekkan Dey, a postdoc in J. Alan Diehl’s group in the Department of Biochemistry at Case Comprehensive Cancer Center, had work accepted for publication in the …