Education
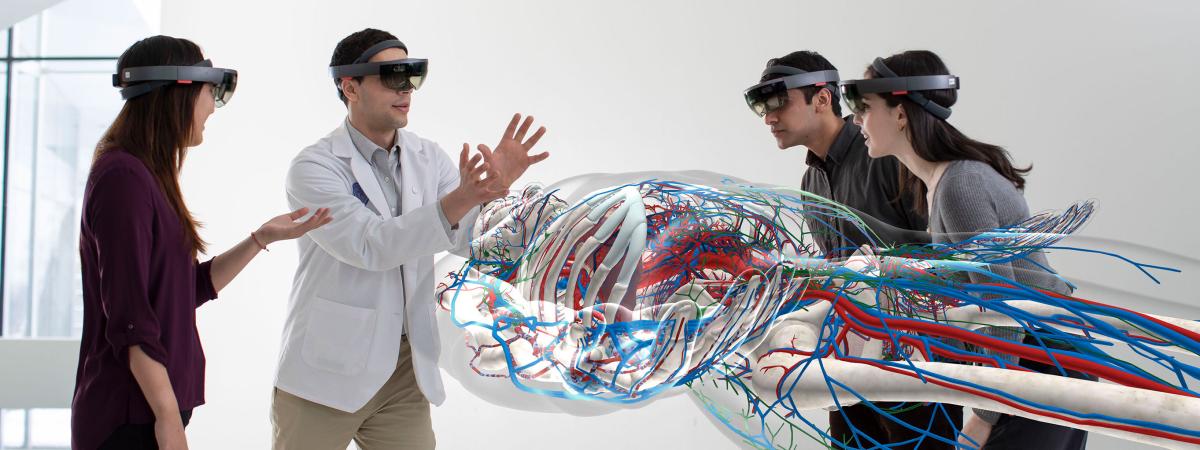
Department of Anatomy faculty are leaders in the creation of new ways of training tomorrow’s doctors, dentists, and physician assistants including the use of augmented reality in learning human anatomy.

MS in Applied Anatomy
In the Master of Science in Applied Anatomy program, our students broaden and deepen their knowledge of basic biology, human health and disease while gaining hands-on experiences to better prepare themselves for a biomedical career.

Research
Faculty are advancing our knowledge of the evolution of mammals and humans, human anatomical variation and functional anatomy, and in medical education.